24+ Why Are Gases Compressible
Web Gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the gas particles. Web And when we look at a liquid we see that theres a little bit more space in between the particles but theyre still not that much space.

14 1 Compressibility Chemistry Libretexts
Web Defines compressibility and gives examples of compressed gases.

. You can directly assign a modality to your classes and set a due date for each class. What is most compressable liquid or. On the other hand volume is effectively utilized in the condensed phases.
Gases are highly compressible than solids and liquids. Interatomic or intermolecular distance in gases is higher than those in liquids. Their molecules are spread out unlike liquids and solids so they can be easily compressed closer to each.
At room temperature and. Web Gases are compressible because of their molecules. Web Gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the gas particles.
Web The volume of molecules increases Z as it makes the pressure higher because the collision frequency is higher. At room temperature and standard pressure the average distance between gas molecules is. Web Gases are compressible because the intermolecular space is very large in gases whereas liquids are not compressible because in liquids the intermolecular space.
This entropic force gives gases a bulk stiffness thats. Web Because most of the volume occupied by a gas is empty space. Web Why gases are compressible but not liquids.
Gases behave this way because. And thus for a gas compression of a given volume simply constrains the gas to a smaller volume in which the volume STILL dwarfs the actual. When we look at a gas we can tell.
Web Gases have the lowest density of the three are highly compressible and completely fill any container in which they are placed. Web Gases are compressible because most of the volume of a gas is composed of the large amounts of empty space between the gas particles. The cohesive forces between molecules.
Medium Solution Verified by Toppr The intermolecular space between the gas particles is very large as compared to the. At room temperature and standard. Web The only thing resisting compression is an entropic force arising from the increasing density as you reduce the volume.
Web gas is compressible because their atoms are spread apart from each other so they can be be moved closer together.

Properties Of Solids Liquids And Gases Youtube

Compressibility Ck 12 Foundation

Properties And The Kinetic Theory Of Gas Britannica
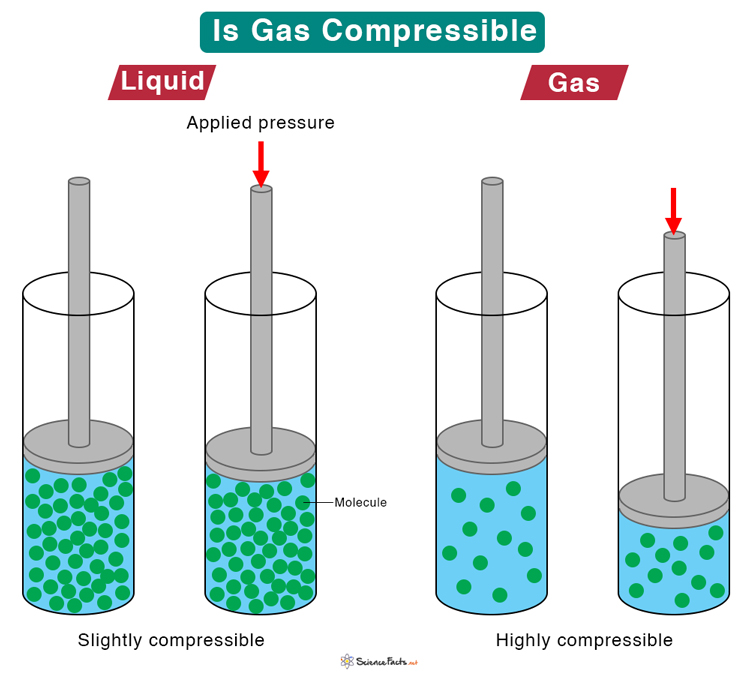
Is Gas Compressible

Gas With Higher Compressibility Factor Will Have More Force Of Attraction B W The Molecules Explain
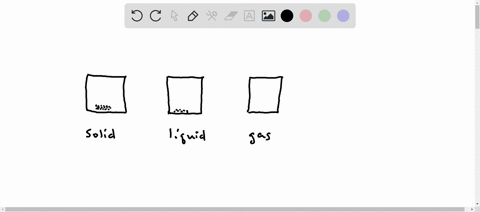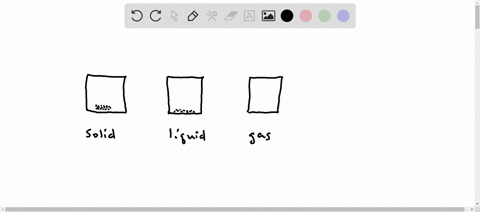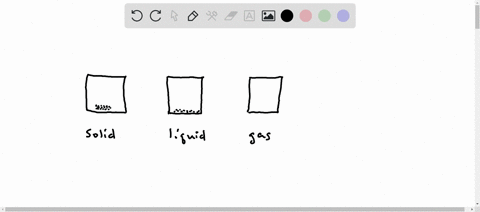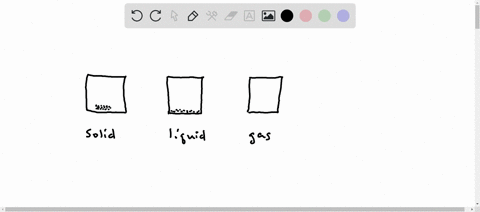
Solved Why Are Gases So Much More Compressible Than Solids Or Liquids
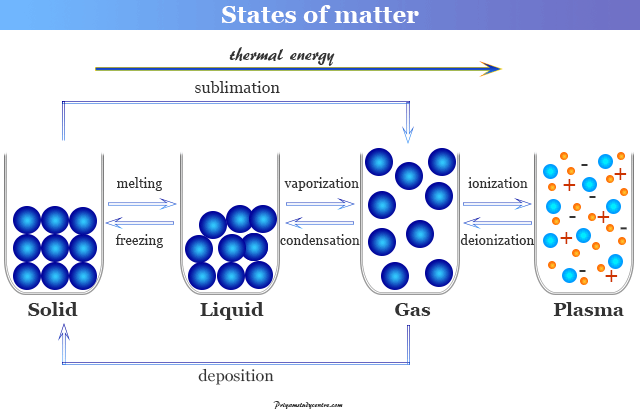
States Of Matter Solid Liquid Gas Plasma

Why Are Gases Highly Compressible
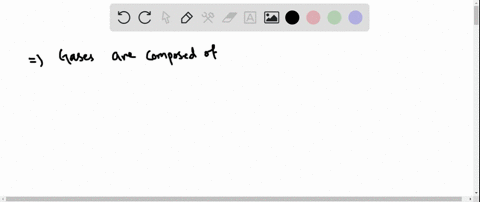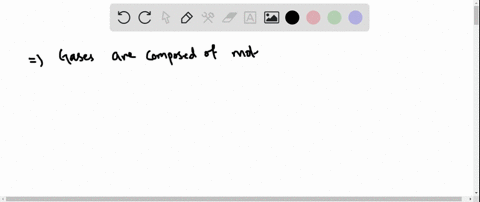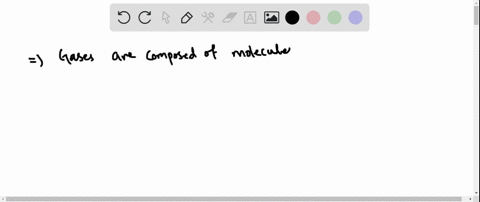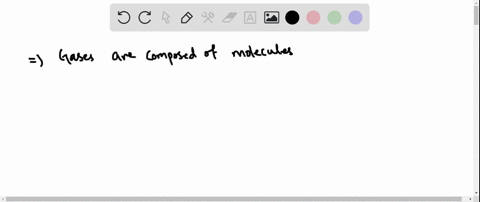
Solved Why Are Gases So Much More Compressible Than Solids Or Liquids

Intermolecular Attractions And The Properties Of Liquids And Solids Chapter Ppt Download

Properties Of Matter Gases Live Science

Solved Gases Are Compressible And Have A Density That Is Chegg Com
Why Are Gas States Compressible But Liquid States Are Not Quora
Why Can Gases Be Compressed Quora
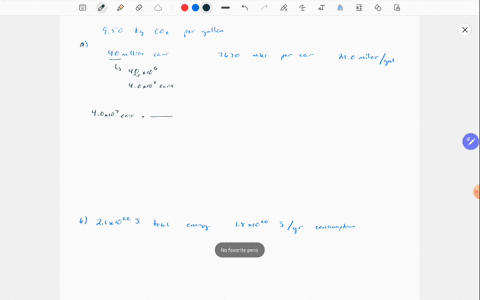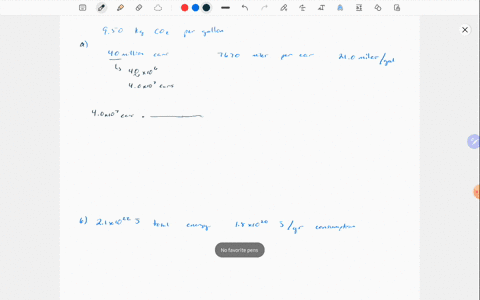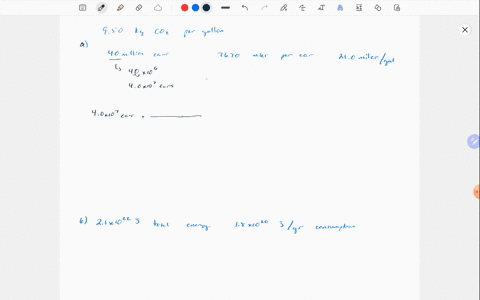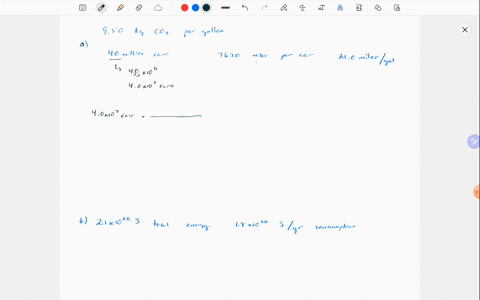
Solved Why Are Gases So Much More Compressible Than Solids Or Liquids

The Behavior Of Gases Chapter 14 Chapter 14 Terms To Know Compressibility Boyle S Law Charles S Law Gay Lussac S Law Combined Gas Law Ideal Gas Constant Ppt Download

Why Are Gases Highly Compressible